
In an earlier era in the US pharma industry, “supply chain” and “logistics” were an afterthought. Pharma companies maintained large warehouses; stocked lots of product;… Read more »

In an earlier era in the US pharma industry, “supply chain” and “logistics” were an afterthought. Pharma companies maintained large warehouses; stocked lots of product;… Read more »
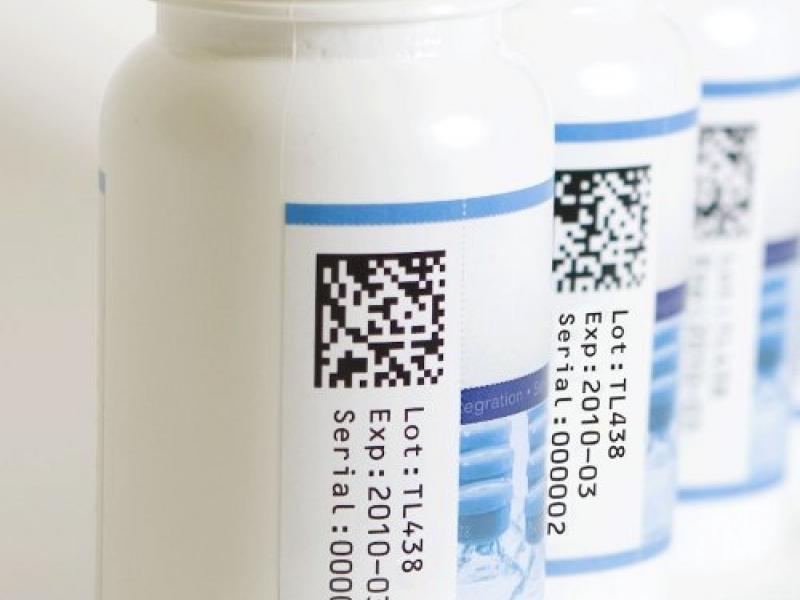
On Nov. 27, 2013 President Obama signed the Drug Quality and Security Act into law, effectively setting the framework of what would become a nationwide initiative toward wide-scale pharmaceutical serialization. The Drug Supply Chain Security Act is one component of the DQSA. This particular act requires the FDA to implement a national track-and-trace system by which manufacturers must affix product identifiers (barcodes) to each package of product that is introduced into the supply chain. Read the full article…

Successfully transporting commodities across the world involves efficient and cost-effective storage and distribution processes. However, another element of successful supply chains requires that environmental specifications for every product are managed and monitored throughout the storage and distribution lifecycle. Read the full article…
MD Logistics designs customized supply chain solutions for global distribution of trade, sample and clinical specialty products. Our pharmaceutical warehouse facilities are fully licensed and… Read more »