Pharma and medical device buyers’ heavy reliance on global sourcing lowers costs but has created supply problems during the coronavirus pandemic. Read the full article…

Pharma and medical device buyers’ heavy reliance on global sourcing lowers costs but has created supply problems during the coronavirus pandemic. Read the full article…
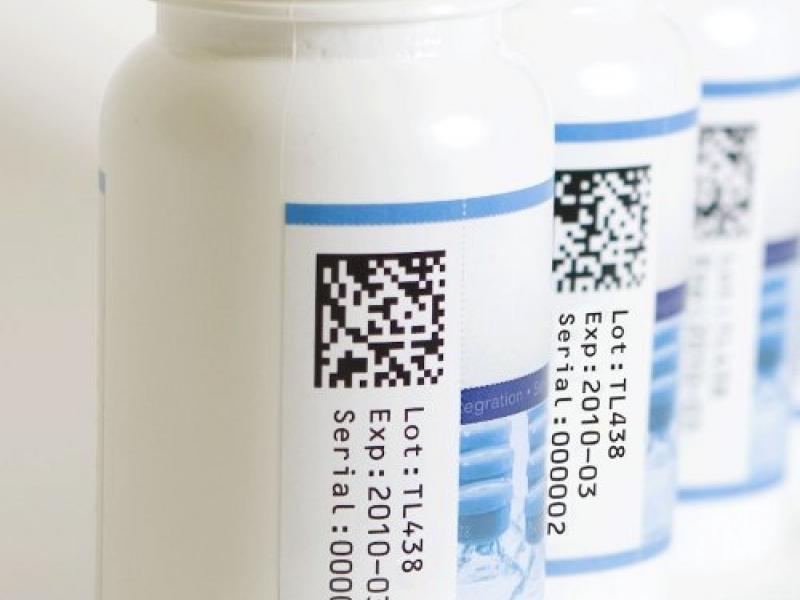
On Nov. 27, 2013 President Obama signed the Drug Quality and Security Act into law, effectively setting the framework of what would become a nationwide initiative toward wide-scale pharmaceutical serialization. The Drug Supply Chain Security Act is one component of the DQSA. This particular act requires the FDA to implement a national track-and-trace system by which manufacturers must affix product identifiers (barcodes) to each package of product that is introduced into the supply chain. Read the full article…

MD Logistics is proud to support a leading event in the life science industry for cold storage and third party logistics providers – the 13th… Read more »

Introduction | Printer Friendly PDF Every year an estimated 5 to 20 percent of Americans contract influenza, resulting in approximately 200,000 hospitalizations. While several factors… Read more »

Successfully transporting commodities across the world involves efficient and cost-effective storage and distribution processes. However, another element of successful supply chains requires that environmental specifications for every product are managed and monitored throughout the storage and distribution lifecycle. Read the full article…

BACKGROUND | Printer Friendly PDF Global trade bypasses international borders and cultural hurdles to unite countries by providing valuable resources and commodities to the people who… Read more »
MD Logistics designs customized supply chain solutions for global distribution of trade, sample and clinical specialty products. Our pharmaceutical warehouse facilities are fully licensed and… Read more »