Written by: Chad Hodges, Director, Pharmaceutical Operations, MD Logistics
In an effort to combat the world of counterfeit drugs and keep patient safety top of mind, The Federal Drug Administration enacted the Drug Supply Chain Security Act (DSCSA), a phased approach to pharmaceutical serialization.
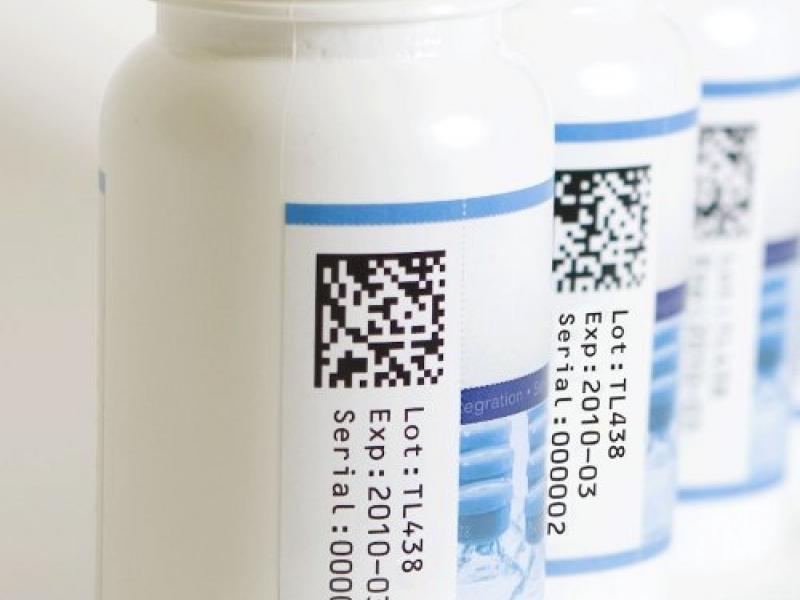
This phased approach, brings with it deadlines to complete product tracking with the end goal being each unit of medication will be able to be traced from the point of origin to the end consumer. By November of this year, pharmaceutical manufacturers must include a unique product identifier on prescription drug packaging and cases. This unique identifier includes the product’s lot number, expiration date, national drug code, and a serial number, making it easily identifiable throughout the course of its shelf life. In addition to making product easily trackable, pharmaceutical serialization also allows officials to better detect illegitimate products throughout the supply chain[1].
Approach
For MD Logistics, our approach to pharmaceutical serialization began in 2008 when we started the conversation with one of our customers. By 2016, our conversation had grown more in depth to the point where we were building out a plan based on their needs and the requirements of the DSCSA legislation. In addition to needing to document where every unit was manufactured, we also needed to capture all serial numbers down to the unit on their way into our building. We were given all serial numbers in a unit, inner pack, case, pallet hierarchy from our customer and had to ensure they were received into our warehouse management system (WMS), JDA/Red Prairie. Tracking unit movement from manufacturer through to the end consumer is a huge element of the DSCSA legislation. Through our integration, we needed to make sure our WMS was tracking this internally and then also pushing that data out to our customers’ third party solution which was tracking the entire life of the product. We did this by building serialized messages to communicate to our customers’ system what product had been received into our warehouse and subsequently shipped out.
To conceptualize what we needed to do as a 3PL provider, my team and I worked alongside our customer to develop a test environment which included all the elements that we were expected to carry out in our solution. Through this test environment we were able to collaborate with our customer, identify potential bottlenecks, and trouble shoot issues that arose prior to implementation. Once testing was complete, we began capturing serial numbers in our live WMS environment.
Expectation vs. Reality
Our early research indicated there wasn’t a provider who had implemented a solution to this magnitude before. We realized that industry benchmarks and procedures were not available for reference, and we would need to rely on our own testing experience to develop serialized processes. While it was easy to conceptualize what we wanted this process to look like, the actual reality of the situation is that once we took our pharmaceutical serialization solution ‘live’, there was an immediate need for additional training and personnel. We had to hire additional team members just to analyze the shear influx of data that we now were tasked with handling.
We had to upgrade our IT systems in order to handle the influx of information that was entering our WMS with every delivery to our warehouse. We also had to upgrade from a paper to digital auditing system. Additionally, our operations team had to learn to use new RF equipment.
Challenges & Learning Experiences
With a project of this magnitude comes a great deal of challenges and learning experiences. We ran into challenges as it related to our existing IT systems and operations processes. In addition to the system upgrades, we had to make several updates to the technology we used on the warehouse floor. We added printers and RF guns to make our processes as efficient as possible to ensure the speed at which orders left the building went unaffected. The emphasis that we placed on inventory control grew exponentially.
Through all the challenges came great learning experiences. Our team learned to adapt and remain nimble throughout all phases of the project. Our IT teams had to adapt to the changes that needed to be made to existing systems and code within our WMS system. Our operations teams had to undergo training to learn the proper way to receive serialized product into the warehouse and the WMS. For many of our warehouse associates, they had to learn how to use new technology. In addition to learning and adapting to a new ‘normal’, our entire operations team worked together to make sure all customer orders left our facility in a timely manner.
A project of this magnitude is not accomplished without the entire company coming together to lend their expertise and a helping hand where needed. Within the company, there were a number of individuals who put in long hours during the week and on the weekends to make sure the transition to a serialized solution was as smooth as possible and our customers remained unaffected.
Conclusion
The MD Logistics Team rose to the challenge of implementing a new solution for our customer, a solution in which few 3PL’s had embarked on previously. As a result of our hard work, our customer is now not only compliant with the November 2018 pharmaceutical serialization requirements, they are now ready for the package-level tracing requirements expected to be implemented in 2023[1]. Throughout this whole process, my admiration of our team has grown. Together we have much to be proud of.
References
[1] “FDA Issues Draft Guidance: Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance Policy”. https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/ucm565358.htm
[2] “Drug Supply Chain Security Act: Overview of Product Tracing Requirements”. https://www.fda.gov/downloads/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/UCM464907.pdf
